Phosphorus (P) is an essential element for all life forms and is stored primarily in soil and sediment. Phosphorus is an essential component of adenosine triphosphate (ATP), which transports chemical energy within cells for metabolism (i.e. uptake and transport of nutrients); deoxyribonucleic acid (DNA), which is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms; and ribonucleic acid (RNA), which is important for protein synthesis in plants and animals.
Phosphorus occurs in soil as inorganic and organic P compounds. Most soils contain a relatively low amount of total P, and only a small fraction of the total P is available to plants. Most P compounds in soils have low water solubility. Once in the soil solution, soluble P moves mainly by diffusion. Phosphorus in soils generally occurs as the anions H2PO4-or HPO42-. Phosphorus reacts with calcium (Ca2+), magnesium (Mg2+), iron (Fe3+), and aluminum (Al3+). Phosphorus reactions in soil are pH dependent. In acid soils, soluble phosphorus in the soil solution reacts with Fe and Al to form low solubility Fe and Al phosphates. In calcareous soils, soluble phosphorus in the soil solution reacts with Ca to form low solubility Ca phosphates.
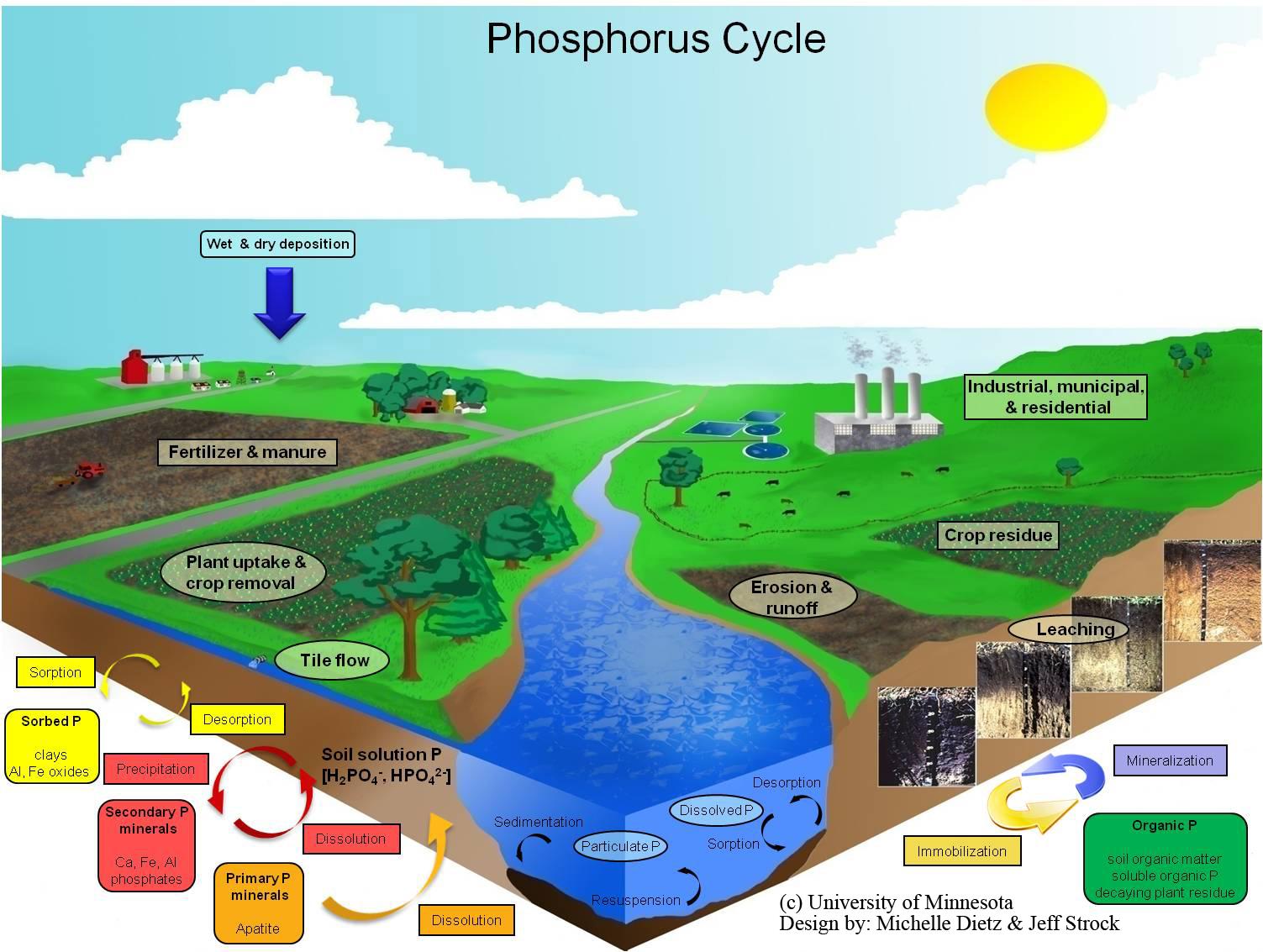
The Phosphorus Cycle is the biogeochemical cycle that describes the transformation and translocation of phosphorus in soil, water, and living and dead organic material. Phosphorus additions to soil occur due to additions of inorganic and organic (manure) fertilizer and the degradation and decomposition of organic (plant and animal) material. Export of P from soil occurs mainly through plant uptake. Phosphorus may also be exported from soil via surface runoff and erosion or subsurface loss through leaching. Sorption and desorption reactions of P occurs on the surfaces and edges of hydrous oxides, clay minerals, and carbonates. Sorption generally occurs by covalent bonds of P with Fe and Al in acidic soils and calcium carbonate (CaCO3) in alkaline soils. Precipitation and dissolution reactions greatly influence the availability of P in the soil. Dissolution of P minerals occurs when P minerals dissolve over time and replenish the P in the soil solution. This reaction increases the availability of P. On the other hand, precipitation occurs when P minerals form by removing P from soil solution. This reaction decreases the availability of P. Precipitation and dissolution are very slow processes. Dissolution and precipitation of P can also occur due to changes in red-ox potential caused by seasonal or periodic waterlogging and draining soil. The microbial cycling of P from inorganic soluble forms to insoluble organic forms is known as immobilization. The reverse is known as mineralization. Mineralization of P is catalyzed by the phosphatase enzyme.